- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Cocaine Hydrochloride |
 |
(Ph. Eur. monograph 0073)
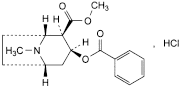
C17H21NO4,HCl 339.8 53-21-4
Local anaesthetic.
Adrenaline and Cocaine Intranasal Solution
Ph Eur
Methyl (1R,2R,3S,5S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate hydrochloride.
98.5 per cent to 101.0 per cent (dried substance).
White or almost white, crystalline powder or colourless crystals.
Very soluble in water, freely soluble in alcohol, slightly soluble in methylene chloride.
About 197 °C, with decomposition.
First identification B, D.
Second identification A, C, D, E.
A. Dissolve 20.0 mg in 0.01 M hydrochloric acid and dilute to 100.0 mL with the same acid. Dilute 5.0 mL of the solution to 50.0 mL with 0.01 M hydrochloric acid. Examined between 220 nm and 350 nm (2.2.25), the solution shows 2 absorption maxima, at 233 nm and 273 nm. The specific absorbance at 233 nm is 378 to 402.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison Ph. Eur. reference spectrum of cocaine hydrochloride.
C. Dissolve 0.1 g in 5 mL of water R and add 1 mL of dilute ammonia R2. A white precipitate is formed. Initiate crystallisation by scratching the wall of the tube with a glass rod. The crystals, washed with water R and dried in vacuo, melt (2.2.14) at 96 °C to 99 °C.
D. It gives reaction (a) of chlorides (2.3.1).
E. It gives the reaction of alkaloids (2.3.1).
Dissolve 0.5 g in water R and dilute to 25 mL with the same solvent.
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
To 10 mL of solution S add 0.05 mL of methyl red solution R. Not more than 0.2 mL of 0.02 M sodium hydroxide is required to change the colour of the indicator.
- 70 to - 73 (dried substance).
Dissolve 0.50 g in water R and dilute to 20.0 mL with the same solvent.
To 0.2 g add 2 mL of sulfuric acid R. After 15 min, the solution is not more intensely coloured than reference solution BY5 (2.2.2, Method I).
Examine by liquid chromatography (2.2.29).
Test solution Dissolve 25.0 mg of the substance to be examined in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (a) Dilute 1.0 mL of the test solution to 50.0 mL with the mobile phase. Dilute 5.0 mL of this solution to 100.0 mL with the mobile phase.
Reference solution (b) Dissolve 25 mg of the substance to be examined in 0.01 M sodium hydroxide and dilute to 10.0 mL with the same solvent. Dilute 1.0 mL of the solution to 10.0 mL with 0.01 M sodium hydroxide. Allow the solution to stand for 15 min.
- — size: l = 0.15 m, Ø = 4.6 mm,
- — stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 µm) with a specific surface area of 335 m2/g, a pore size of 10 nm and a carbon loading of 19.1 per cent,
- — temperature: 35 °C.
Mobile phase triethylamine R, tetrahydrofuran R, acetonitrile R, water R (0.5:100:430:479.5 V/V/V/V).
Flow rate 1 mL/min.
Detection Spectrophotometer at 216 nm.
Injection 20 µL.
Relative retention With reference to cocaine (retention time = about 7.4 min): degradation product = about 0.7.
System suitability Reference solution (b):
- — resolution: minimum of 5 between the peaks due to cocaine and to the degradation product.
- — any impurity eluting after the principal peak: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent),
- — total: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent),
- — disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Maximum 0.1 per cent, determined on the residue from the test for loss on drying.
Dissolve 0.250 g in a mixture of 5.0 mL of 0.01 M hydrochloric acid and 50 mL of alcohol R. Carry out a potentiometric titration (2.2.20), using 0.1 M sodium hydroxide. Read the volume added between the 2 points of inflexion.
1 mL of 0.1 M sodium hydroxide is equivalent to 33.98 mg of C17H22ClNO4.
Protected from light.

A. methyl (1R,2R,3S,5S)-8-methyl-3-[[(E)-3-phenylpropenoyl]oxy]-8-azabicyclo[3.2.1]octane-2-carboxylate (cinnamoylcocaine),

B. bis[(1R,2R,3S,5S)-2-(methoxycarbonyl)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl] (1r,2c,3t,4t)-2,4-diphenylcyclobutane-1,3-dicarboxylate (α-truxilline),

C. bis[(1R,2R,3S,5S)-2-(methoxycarbonyl)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl] (1r,2c,3t,4t)-3,4-diphenylcyclobutane-1,2-dicarboxylate (β-truxilline).
Ph Eur

