INTRODUCTION
Definition and Scope
The chapter is part of a group of general information chapters for immunological test methods (Immunological Test Methods—General Considerations  1102
1102 , Immunological Test Methods—Enyzme-Linked Immunosorbent Assay (ELISA)
, Immunological Test Methods—Enyzme-Linked Immunosorbent Assay (ELISA)  1103
1103 , and Immunological Test Methods—Surface Plasmon Resonance
, and Immunological Test Methods—Surface Plasmon Resonance  1105
1105 ) that provides analysts with general information about principles, method development, method validation, and data evaluation for immunoblot analysis. Immunoblot analysis is defined as any method in which an antibody is used for detection of one or more analytes (e.g., proteins, polysaccharides) that has been transferred to a test membrane surface. Immunoblot methods are typically classified by whether electrophoretic separation occurs as a part of the immunoblot procedure. Electrophoretic separation is based on molecular weights and charge differences of a population of molecules. See Electrophoresis
) that provides analysts with general information about principles, method development, method validation, and data evaluation for immunoblot analysis. Immunoblot analysis is defined as any method in which an antibody is used for detection of one or more analytes (e.g., proteins, polysaccharides) that has been transferred to a test membrane surface. Immunoblot methods are typically classified by whether electrophoretic separation occurs as a part of the immunoblot procedure. Electrophoretic separation is based on molecular weights and charge differences of a population of molecules. See Electrophoresis  726
726 , Capillary Electrophoresis
, Capillary Electrophoresis  1053
1053 , Biotechnology-Derived Articles—Isoelectric Focusing
, Biotechnology-Derived Articles—Isoelectric Focusing  1054
1054 , and Biotechnology-Derived Articles—Polyacrylamide Gel Electrophoresis
, and Biotechnology-Derived Articles—Polyacrylamide Gel Electrophoresis  1056
1056 for a detailed description of electrophoretic separation methods. An example of immunoblot analysis involving electrophoretic separation is the Western blot, which was first described in the scientific literature in the late 1970s. Another approach for immunoblot analysis is to perform molecule detection using an antibody without prior electrophoretic separation. Examples of this nonelectrophoretic type of approach are the slot or dot blot (slot/dot).
for a detailed description of electrophoretic separation methods. An example of immunoblot analysis involving electrophoretic separation is the Western blot, which was first described in the scientific literature in the late 1970s. Another approach for immunoblot analysis is to perform molecule detection using an antibody without prior electrophoretic separation. Examples of this nonelectrophoretic type of approach are the slot or dot blot (slot/dot).
The scope of this chapter includes only those methods in which an antibody is used for the detection of a molecule bound to a membrane. Therefore this chapter does not discuss procedures that use nonimmunological means of detection.
ASSAY SELECTION
Nonelectrophoretic Assay (Slot/Dot Blot)
The slot/dot blot method is a simplified, nonelectrophoretic method in which a mixture containing the analyte(s) for detection is first applied directly to the membrane using a vacuum manifold machined to contain regularly spaced rectangular slots. The slot/dot blot method is faster and simpler because there is no electrophoretic separation of the multiple, individual analytes that may be present in the mixture. For these reasons, it can be readily adapted for automated analysis of multiple samples, for which a number of systems are commercially available, but it offers no information about the molecular weight and only limited information regarding the quantity of sample. Although it can be set up in a quantitative format, the method usually is used to produce a qualitative result, e.g., confirming identity by demonstrating the presence or absence of specific antigens by means of an immunocomplex detection system. After the analytes are bound to the membrane and unbound sites are blocked, analysts use a detector antibody to determine the presence or absence of the analyte or analytes of interest. The uniform shape of the slot blot and its greater surface area for analyte binding make it better suited than the dot blot for quantitative applications and analysis by densitometry.
Electrophoretic Assay
Electrophoretic blotting methods (commonly called Western blots) are widely used for analyzing mixtures of proteins. The Western blot is a powerful tool to study the identification, relative concentration, relative molecular weight, and posttranslational modifications of specific proteins. In Western blots, the proteins of the sample are separated using gel electrophoresis. Protein separation may be based on molecular weight alone or on isoelectric point (pI) and molecular weight. Proteins migrate either in one dimension (1D) or in two dimensions (2D) through a gel. When proteins are separated by their molecular weights, the smaller proteins migrate faster and separate according to molecular weight. When analysts use a 2D gel, proteins are separated according to pI in the first dimension, and then according to their molecular weights in the second dimension. After separation, the proteins are transferred to a membrane, the membrane is blocked to avoid nonspecific binding of subsequent assay reagents, and the protein of interest is detected using specific antibodies.
A bound antibody can be detected by different methods, including colorimetric detection, fluorescent detection, chemiluminescent detection, and radioactive detection. Upon detection of the protein(s) of interest, immunoblot quantitation can be indirectly performed by densitometry.
1d electrophoresis
In 1D electrophoresis, individual proteins or groups of proteins are separated by molecular weight for further analysis by Western blot. Using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), proteins migrate in response to differences in electrical charge through a 3D network of fibers and pores. The network is formed as the bifunctional bisacrylamide, or other cross-linker, cross-links adjacent polyacrylamide chains to form a gel (see also USP general chapter  1056
1056 ). The combination of gel pore size and protein characteristics determines the migration of proteins. Separated proteins are detected subsequently by Western blot analysis using antibodies specific to the target proteins. By means of Western blot analysis, a test sample can be compared to a standard, and the appearance of degradants and impurities specifically related to the target proteins can be monitored if the detection antibody can still recognize the altered forms of the protein. Although a high level of sensitivity can be achieved by this approach, separation of individual proteins at similar molecular weights may not be achieved. If analysts must probe individual proteins at similar molecular weights, 2D separations may be required.
). The combination of gel pore size and protein characteristics determines the migration of proteins. Separated proteins are detected subsequently by Western blot analysis using antibodies specific to the target proteins. By means of Western blot analysis, a test sample can be compared to a standard, and the appearance of degradants and impurities specifically related to the target proteins can be monitored if the detection antibody can still recognize the altered forms of the protein. Although a high level of sensitivity can be achieved by this approach, separation of individual proteins at similar molecular weights may not be achieved. If analysts must probe individual proteins at similar molecular weights, 2D separations may be required.
2d electrophoresis
In 2D electrophoresis, individual proteins or groups of proteins are separated in the first dimension by isoelectric focusing (IEF; charge) and in the second dimension by electrophoresis in the presence of SDS (molecular weight). Separating proteins this way allows information to be obtained not only about molecular weight, as in 1D gels, but also about the charge of a protein. Two-dimensional gels are a useful choice for resolving complex mixtures and for assessing protein antibody specificity (e.g., evaluation of host cell proteins).
Membrane, Reagent, and Detection Options
membranes
Generally, both nitrocellulose and polyvinylidine fluoride (PVDF) membranes are used for immunoblot methods. For cost considerations, nitrocellulose membranes are often preferred over PVDF membranes for slot/dot blots (or vacuum blotting), but due to their greater mechanical strength, PVDF membranes should be considered if stripping and reprobing are required.
blocking reagents
Following transfer or binding of protein to membranes, the unoccupied binding sites on the membranes must be blocked to prevent nonspecific binding of subsequent reagents. Most detection probes are proteins that also can bind to the membrane. Failure to appropriately block the membrane sites can result in nonspecific binding and high background. A number of blocking reagents are available, including gelatin, nonfat milk, and bovine serum albumin (BSA). Proteins should be unrelated to the antigens used in the study. Because these reagents often have lot-to-lot variability, they may require qualification. They must be evaluated with the detection system selected and optimized using that detection system for minimal background with no loss of signal. If the blocking reagent is derived from a biological source, it must not contain trace levels of the protein under measurement, because the latter can increase the background.
methods of detection
Immunological detection of analytes in any type of immunoblot can be direct or indirect. The choice of format depends on a combination of the level of sensitivity required and the quality of the antisera available. For identity or product detection, sensitivity usually is not critical, and direct detection via a conjugated antibody is commonly used, which often simplifies and shortens the time required to execute the method. Alternatively, indirect detection, usually by the use of a conjugated anti-species reagent, can be used to improve sensitivity. On some occasions, the analyte being detected is actually an antibody, as in the case of a monoclonal antibody that is being developed as a drug. In this case, antibodies specific for the antibody (e.g., anti-idiotypic antibodies) can be used.
Primary antibody:
The primary antibody is selected based on its specificity for the analyte or protein. Although polyclonal anti-sera can offer a broad range of detection against a potentially large set of epitopes, an unwanted cross-reaction resulting in decreased specificity may occur. If this cannot be overcome by assay optimization, a monoclonal antibody or groups of selected monoclonal antibodies can be used. Monoclonal antibodies are often advantageous for long-term studies, because they yield a consistent supply of antibody against a specific epitope. The use of monoclonal antibodies directly limits the number of epitopes involved in the detection of the target. This must be evaluated for each application. The primary antibody may be directly conjugated or used in conjunction with a secondary antibody and an appropriate detection system. The optimal antibody concentration usually is considered to be the greatest dilution of antibody that results in a strong positive signal with minimal background. This must be optimized in conjunction with the block and detection system selected. The primary antibody should be qualified before assay use.
Secondary antibody:
The secondary antibody typically is directed against the species of the primary antibody immunoglobulin (which is specific for the analyte, e.g., goat anti-mouse IgG). Enzymes such as horseradish peroxidase (HRP) or alkaline phosphatase (AP) typically are linked to the secondary antibody, but other labels such as fluorophores or gold particles can be used for detection. If the secondary antibody is biotinylated, biotin–avidin–HRP or –AP complexes can be used for detection.
Detection enzyme and substrate:
Once an immunocomplex containing the enzyme–conjugate reagent has formed, analysts add a suitable substrate to the assay. This reaction results in production of a colored precipitate or a fluorescent or chemiluminescent product that can be recorded, measured, and analyzed further. A broad range of detection options is available to best fit individual applications and intended uses. A number of these are described in  1103
1103 and
and  1102
1102 , as well as in Table 1.
, as well as in Table 1.
Table 1. Detection Reagents and Methods
| Readout | Principle of the Enzymatic Reaction |
Enzyme | Substrate | Detection | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Colorimetric | Produces a colored product that yields absorbance values directly proportional to analyte concentration | APa
HRPc |
pNPPb
TMBd OPDe ABTSf |
Spectrophotometer | — Robust — Economical — Reagent availability |
— Time-consuming — Less sensitive than other methods |
| Chemiluminescent | Produces a light emission that is directly proportional to analyte concentration | AP, HRP | CSPDg | Luminometer, photographic film (CCDh camera) | —Wide assay dynamic range — Very sensitive — Rapid signal generation |
— Reproducibility can be challenging |
| Fluorescent | Produces excitation-induced light emission that is directly proportional to analyte concentration | Galactosidase, fluorescently labeled antibody | MGi
NGj |
Fluorometer (CCD camera with filters) | — Rapid — Sensitive |
— Interference by excipients |
| Radioactive | Antigen is labeled with a radioactive isotope. Radiation is proportional to analyte concentration. | — | — | Scintillation counter | — Easy to quantitate — Rapid |
— Safety risk with exposure — Radioactive waste |
|
a
Alkaline phosphatase.
b
para-Nitrophenyl phosphate.
c
Horseradish peroxidase.
d
3,3',5,5'-Tetramethylbenzidine.
e
o-Phenylenediamine dihydrochloride.
f
2,2'-Azino-bis[3-ethyl-benzothiazoline-6-sulfonic acid]diammonium salt.
g
Disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2'-(5'-chloro)tricyclo[3.3.1.13,7]decan}-4-yl)phenyl phosphate.
h
Charge-coupled device.
i
4-Methylumbelliferyl galactoside.
j
Nitrophenyl galactoside.
|
||||||
METHOD DEVELOPMENT
Method development can proceed on the basis of the background information just provided. The scope of method development, and eventually method validation, are dictated by the purpose of the method. The purpose determines the format for the assay and other requirements for the test, and therefore, the purpose should be determined first. The following sections explore considerations for each method's purpose.
Intended Purpose of the Method
identity testing
In the case of identity tests, analysts want to detect the presence of a protein; therefore, demonstration of specificity is essential and required. For this purpose, analysts also control the quantity of protein in the sample. Thus, the limits of detection (LOD), limits of quantitation (LOQ), and other measures of quantity are not required attributes of the method. Examples include material identity assays that demonstrate the isotype of an IgG and, in some cases, demonstrate the specificity of an antibody in a method validation. If there is no interference from the matrix or potential cross-reaction with other materials present in the sample, then a simple slot/dot blot may suffice. If multiple proteins in the sample display immunoreactivity and must be distinguished from each other, another separation procedure must be used before blotting and immunostaining. The complexity of the proteins in the sample and the usefulness of the additional information gained using an electrophoretic separation help determine if a slot/dot blot can meet the needs of the test.
limit testing
In other applications, analysts may want to show that an impurity has been removed to a level below toxicological concern. In many cases, a limit test is used when it is possible to say yes or no about the presence or absence of a protein below a predetermined level. This simplifies the development and validation of the method. With densitometry (scanning or imaging) equipment, the intensity of spots or bands can be determined relative to a standard curve, resulting in an estimate of concentration. An LOD should be determined to establish the appropriate limit threshold for the method. A dot blot may be suitable for either circumstance if the specificity of the antibody in the sample matrix can be demonstrated.
Another common purpose for an immunoblot is to show the presence or absence of a protein expressed from a culture. In this situation, analysts want to establish the identity of the protein by immunostaining, as well as verify that the protein has the expected molecular weight. This provides further assurance of no nonspecific interactions with other proteins in a complex matrix that generates the signal in the blot.
specificity testing
Characterizing the specificity of the reagents for an ELISA impurity test or an immunoaffinity column also is a common immunoblot purpose. This is another form of an identity test in which the desired endpoint is demonstration of the specificity of binding between the antigen and the antibody. The result of the measurement is a demonstration of binding to a select group of the total protein population in the sample or binding to the whole population of proteins in the sample, as required for host-cell protein assays. In order to demonstrate the specificity of an antibody relative to a population of proteins, analysts typically must carry out electrophoretic or other separations. Showing, by means of immunostaining, that a protein of the right molecular weight or pI can be recognized by the antibody is a powerful demonstration both of specificity toward a given protein and the absence of binding to other proteins. In addition, having, within the same experiment, the appropriate positive control samples that are known to contain the protein, and the appropriate negative samples that are known not to contain the protein, makes a convincing argument for the specificity and selectivity of the antibody when analysts validate an ELISA method for protein impurities. Electrophoretic separations can be done in one dimension using either SDS–PAGE (for molecular weight) or isoelectric focusing (IEF; for isoelectric point) for a limited number of proteins with known molecular weights. Electrophoretic separations also can be done in two dimensions (e.g., IEF followed by SDS–PAGE) to show selectivity and specificity toward a more heterogeneous population of proteins. A 2D Western blot commonly is used to demonstrate the specificity of a polyclonal antibody candidate directed against a host-cell protein (HCP) antigen preparation before development of a quantitative ELISA for that purpose.
Assay Mode and Sample Introduction
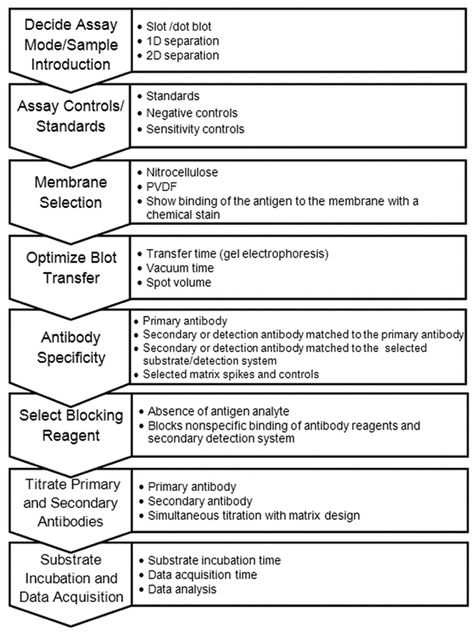
After considering the critical elements required for each purpose of the method, the analyst can use this information to select the most appropriate assay mode. The main points to consider when developing an immunoblot method are shown in Figure 1. If appropriate, spotting samples on a membrane or applying by vacuum is the easiest and most convenient way to introduce a sample to an immunoblot membrane. However, low levels of nonspecific binding from multiple proteins can create additive nonspecific interference in dot blots or slot blots, resulting in background levels that appear to be the desired analyte.
Electrophoretic separations, although time consuming, can be useful for separating and further distinguishing specific and nonspecific binding. Analysts must trade sensitivity for selectivity in going from a single dimension to two dimensions because of the further separation of immunoreactive species from a single band into multiple spots, as is the case with the heterogeneity seen in sialylated proteins or deamidated species.
Assay Controls and Standards
Controls and standards are selected based on the purpose of the assay and the information needed during development. Protein molecular weight markers can be used to obtain an accurate estimate of the molecular weight of immunoreactive species. The use of positive and negative controls is helpful for troubleshooting throughout the experimental design process. Standards or positive and negative controls can be used to assess system suitability and to establish method performance. A positive control can confirm appropriate protein migration and can confirm that membrane transfer has reached completion. A negative control is useful for assessing nonspecific interactions. A method sensitivity control near the LOQ can be used to measure the consistency of the method near the LOQ to evaluate changes in assay performance.
Membrane Selection
A membrane is selected based on the application and the protein being measured. Membranes with various pore sizes should be available and should be suitable for the molecular weight of the protein of interest to aid in appropriate transfer of different sizes of proteins. If a chemical staining method is known to work on a specific membrane with a specific protein, then it is advantageous to show that the protein binds to the membrane and is stained before analysts work on the immunostaining steps for the assay. Electrophoretic separations followed by transfer to a membrane should be optimized with chemical staining, e.g., with sensitive fluorescent stains or silver stains and at potentially higher loads before analysts work on lower load levels required for blot optimization. Stains such as Coomassie or colloidal Coomassie may not have sufficient sensitivity to detect a low level of impurities required for certain applications.
Optimize Blot Transfer
Analysts should optimize transfer times from the gel to the membrane. Larger proteins require more time for transfer than smaller proteins. Small proteins may be lost during long transfer times and can transfer from the gel all the way through the membrane and be lost on the other side. The density of the gel and gradient gels can result in nonuniformity of transfer from the top to the bottom of the gel. During transfer optimization, many method developers use multiple membranes in order to capture proteins that transfer through the first membrane. Chemical staining of both the gel and the membranes can provide useful information about the location of the proteins transferred from the gel to support, either extending or reducing the transfer time.
After they select the assay mode, analysts next investigate spotting of the antigen or transfer from a gel to the appropriate membrane at various relevant concentration levels. Levels of analyte above the concentration needed for a Western blot may be required at first to determine if transfer and recognition by the antibodies is possible. If the analyte is present in low concentrations, spiking may be necessary to show its location during transfer optimization. Because of the potential variability of immunostaining and transfer, a sensitivity control or several levels of controls should be incorporated into the method based on the analyte titration above the background level. This can be adjusted as method development progresses.
Antibody Specificity
Analysts should demonstrate antibody specificity early in immunoblot method development. If possible, they should test samples of the matrix without the analyte and should show an absence of response. In contrast, samples that contain analyte spiked into the matrix should show a positive response, demonstrating the specificity of the antibodies.
Analysts also should demonstrate the specificity of the secondary antibody conjugate or label. Control immunoblots with lanes or spots of primary antibody and control matrix samples containing the analyte as a negative control can show that the secondary antibody is binding to the primary antibody and not to proteins found in the matrix. Commercial sources for enzyme conjugates or fluorescent-labeled anti-species antibodies are readily available and normally are screened or affinity purified against the species of antibody being detected, which eliminates some of the early work needed to achieve the desired specificity. The secondary antibody or detection system must be matched with the detection equipment and the desired sensitivity of the assay, e.g., fluorescence, colorimetric precipitating substrates, or chemiluminescence.
Select Blocking Reagent
Replicate membranes can be blocked with previously described blocking agents as analysts select the most appropriate blocking reagent and the amount of time required to minimize background by means of subsequent primary and secondary antibody incubations. Analytes titrated at multiple concentrations on the membrane allow analysts to assess the amount of signal to the amount of noise (background) with various blocking reagents followed by immunostaining with the primary antibody, labeled secondary antibody, and substrate, if needed, for visualization. This titration also serves as the starting point for examining LOD and LOQ for limit tests and quantitative measurements. The LOD for immunoblots is determined by the level of nonspecific background relative to the specific signal from the analyte. As is the case with any other analytical method, if the background and signal are equal, there is no distinction between the signal and the noise.
Titrate Primary and Secondary Antibodies
Titrating the level of primary and secondary antibody from low to high dilutions can also, as with a blocking reagent, be used to select an antibody concentration that reduces background binding in the blank regions surrounding protein spots or bands, and can optimize the signal from the analyte. A matrix grid that varies the level of primary signal against secondary signal can be useful for optimizing the background, improving analyte signal, and reducing the consumption requirements for expensive antibody reagents.
Immunoaffinity chromatography against a highly purified antigen can be used to reduce the level of nonspecific interference for all of the immunological reagents used in an immunoblot. The method developer must be cautious that the selectivity, specificity, and affinity of the primary antibody are not lost in affinity purification because of high-affinity antibodies that remain on the antigen column or because of the destruction of antibody binding caused by elution conditions. For the secondary antibody, immunoaffinity-purified anti-species antibodies are available commercially with a variety of possible labels conjugated to the antibody.
Substrate Incubation and Data Acquisition
Analysts can optimize substrate development time for enzymes in order to minimize background and improve the LOD and LOQ. Excessive substrate development times for precipitating substrates can result in an intensification of the background level relative to the specific signal from the desired analyte. If the blot is agitated during substrate incubation, undesired swirling patterns of product from precipitating substrates can form. Too short an incubation time results in a less-specific signal, but too long a time can result in high background and poor resolution. Most enzyme conjugates have an optimum development time. Fluorescent labels and chemiluminescent labels have the advantage of acquisition by scanning instrumentation that can store data electronically and perhaps acquire image signals in an additive manner. Fluorescent labels have the added advantages that the signal is stable with time, numerous experiments for development time can be obtained with a single blot, and optimization of signal acquisition can be performed on a single blot.
PROCEDURES
Slot/Dot Blots
Using an appropriate slot/dot apparatus, analysts can make antigens of interest adhere to a suitable membrane (e.g., nitrocellulose) by gravity or vacuum filtration, followed by addition and incubation of antigen-specific antibodies that bind to epitopes on the antigens. Remaining binding sites on the membrane are blocked by the addition of nonspecific antigen (e.g., BSA), followed by probing of the antigen-specific antibodies with a detection system [e.g., protein A/G conjugated to HRP binds to the antibodies that then are visualized using a 4-chloro-naphthol (4-CN) peroxidase substrate]. Positive identification is the development of dots or bands on the membrane. A negative result remains white or exhibits faint bands that are considerably lighter than positive bands.
1D Immunoblotting
preparation of sds–page gels
Analysts should choose an SDS–PAGE gel with a content of acrylamide–bisacrylamide suitable for the molecular weight(s) of the protein(s) of interest; i.e., the smaller the molecular weight of the protein, the higher the percentage of mono- or bisacrylamide, and conversely, the larger the molecular weight of the protein, the lower the percentage of mono- or bisacrylamide.
Table 2. Linear Range of Separation (kD) for Uniform-Concentration Gels
| Acrylamide Concentration (%) |
Linear Range of Separation (kD) |
|---|---|
| 5 | 57–212 |
| 7.5 | 36–94 |
| 10 | 20–80 |
| 12 | 12–60 |
| 15 | 10–43 |
Table 3. Linear Range of Separation (kD) for Gradient Gels
| Acrylamide (%) |
Protein Range (kD) |
|---|---|
| 5–15 | 20–250 |
| 5–20 | 10–200 |
| 10–20 | 10–150 |
| 8–20 | 8–150 |
samples and standard
To prepare samples, analysts typically must lyse cells and tissues in order to release the proteins of interest. The main consideration when choosing a lysis buffer is whether the antibody chosen for detection of the protein(s) of interest can recognize denatured samples. When this is not the case, analysts use buffers without detergent or with relatively mild, nonionic detergent.
Samples should be treated (e.g., reduced, nonreduced, or denatured) according to general chapter  1056
1056 , and when a sample of unknown protein content is used, a series of dilutions should be loaded onto the gel. Standards (molecular weight markers) should be treated according to the manufacturer's instructions.
, and when a sample of unknown protein content is used, a series of dilutions should be loaded onto the gel. Standards (molecular weight markers) should be treated according to the manufacturer's instructions.
electrophoresis
Before applying samples to the stacking gel wells according to  1056
1056 , analysts denature samples (e.g., heat at 95
, analysts denature samples (e.g., heat at 95 –100
–100 for 5 min). An appropriate volume of sample is loaded onto the gel, and a voltage of 8 V/cm applied until the dye has moved into the resolving gel. Afterward, the voltage is increased to 15 V/cm, and the separation is run until the bromophenol blue reaches the bottom. If a commercially available gel is used, the manufacturer's recommendations are followed. Table 4 shows common sample-loading volumes for particular gels.
for 5 min). An appropriate volume of sample is loaded onto the gel, and a voltage of 8 V/cm applied until the dye has moved into the resolving gel. Afterward, the voltage is increased to 15 V/cm, and the separation is run until the bromophenol blue reaches the bottom. If a commercially available gel is used, the manufacturer's recommendations are followed. Table 4 shows common sample-loading volumes for particular gels.
Table 4. Common Sample-Loading Volumes
| Wells | Gel Thickness (mm) |
Maximum Sample Load Volume (µL) |
|---|---|---|
| 10 | 1.0 | 25 |
| 10 | 1.5 | 37 |
| 12 | 1.0 | 20 |
| 15 | 1.0 | 15 |
| 15 | 1.5 | 25 |
transfer
After electrophoresis, the proteins of interest can be blotted to a membrane such as nitrocellulose or PVDF with a pore size that is appropriate for the molecular weight of the proteins of interest. Both nitrocellulose and PVDF have a protein-binding capacity of about 100–200 µg/cm2. PVDF is more chemically resistant than nitrocellulose and is easier to handle. Detailed instructions for the transfer process can be found on the websites of the manufacturers of transfer apparatus and vary depending on the system.
Transfer can be done in wet or semi-dry conditions. Semi-dry transfer generally is faster, but wet transfer is especially recommended for large proteins >100 kD. For both kinds of transfer, the membrane is placed next to the gel. The two are sandwiched between absorbent materials, and the sandwich is clamped between solid supports to maintain tight contact between the gel and membrane.
A standard buffer for transfer is the same as the buffer used for the migration or running buffer without SDS, but with the addition of methanol to a final concentration of 20%. For proteins larger than 80 kD, SDS should be included at a final concentration of 0.1%. Lowering methanol in the transfer buffer also promotes swelling of the gel, allowing large proteins to transfer more easily. Table 5 contains common buffers used for Western blot methods.
Table 5. Common Western Blot Buffer Formulations
| Buffer | Content |
|---|---|
| Sample buffer 2× (nonreducing) 1D electrophoresis |
1.89 g of Tris 5.0 g of SDS 50 mg of bromophenol blue 25.0 mL of glycerol 100 mL of water Adjust with HCl to a pH of 6.8. Add water to 125 mL. |
| Sample buffer 2× (reducing) 1D electrophoresis |
To nonreducing sample buffer: Add 12.5 mL of 2-mercaptoethanol before adjusting the pH. Alternatively, use 1.93 g of Tris, and add a suitable quantity of DTTa to obtain a final concentration of 100 mM DTT. |
| Running buffer 10× 1D electrophoresis |
151.4 g of Tris 721.0 g of glycine 50.0 g of SDS Add water to 5000 mL. Adjust to a pH of 8.1–8.8. |
| Transfer buffer 10× | 151.4 g of Tris 721.0 g of glycine Add water to 5000 mL. Adjust to a pH of 8.1–8.8. |
| Transfer buffer 1× | 100 mL of 10× stock 500 mL of water 200 mL of methanol Add water to 1000 mL. |
| TBS 10× | 24.23 g of Tris base 80.06 g of NaCl Mix in 800 mL of ultrapure water. Adjust with pure HCl to a pH of 7.6. Add water to 1000 mL. |
| TBS-T | 100 mL of TBS 10× 900 mL of water 1 mL of polysorbate 20 |
| 8.5 M urea stock | 510 g of urea Add water to 1000 mL. |
| Sample buffer 2D electrophoresis |
47 mL of 8.5 M urea stock 385 mg of tributyl phosphine (TBP) 2 g of CHAPSb 25 mg of bromophenol blue 1% carrier ampholytes of choice |
|
a
Dithiothreitol.
b
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate.
|
|
Methanol is necessary only if analysts use nitrocellulose. If they use PVDF, they can remove methanol from the transfer buffer and need only to activate the PVDF before they assemble the gel and membrane sandwich.
In semidry transfer, a sandwich of paper/gel/membrane/paper wetted in transfer buffer is placed directly between the cathode and anode. During wet transfer the membrane should be closest to the positive electrode, and the gel should be closest to the negative electrode. The composition of the transfer buffer is not necessarily the same as the migration or running buffer. Analysts should consult the apparatus manufacturer's protocol, and it is common to add both SDS and methanol. The balance of SDS and methanol in the transfer buffer, the proteins' molecular weights, and the gel percentage can affect transfer efficiency for both wet and semidry transfers.
blocking
Blocking the membrane prevents nonspecific background binding of the primary and secondary antibodies to the membrane. Traditionally, one of two blocking solutions is used: nonfat milk or BSA (Cohn fraction V). Milk is cheaper but is not recommended for studies of phosphoproteins. To prepare a 5% milk or BSA solution, weigh 5 g/100 mL of Tris-buffered saline containing polysorbate 20 buffer (TBS-T; see Table 5). Mix well, and filter. Failure to filter can lead to spotting in which tiny dark grains contaminate the blot during development. Incubate at 4 for 1 h with gentle shaking. Rinse in TBS-T after the incubation.
for 1 h with gentle shaking. Rinse in TBS-T after the incubation.
primary antibody and incubation buffer
Dilute the antibody with blocking buffer at a proper dilution (1:100–1:3000, depending on antibody titer), and optimize the dilution according to the results. Too much antibody can result in nonspecific bands.
incubation time
Incubation time can vary between a few hours and overnight, and depends on the binding affinity of the antibody for the protein and the abundance of protein. A more dilute antibody with a prolonged incubation may improve specific binding.
incubation temperature
It is best to incubate under cold temperatures. When analysts incubate in blocking buffer overnight, they should incubate at 4 to prevent contamination from bacterial growth, and should gently agitate the antibody solution to enable adequate homogeneous covering of the membrane.
to prevent contamination from bacterial growth, and should gently agitate the antibody solution to enable adequate homogeneous covering of the membrane.
secondary antibody and incubation buffer
Handle the secondary antibody and incubation buffer as follows. Wash the membrane several times in TBS-T while agitating to remove residual primary antibody. Dilute the secondary antibody with TBS-T at the suggested dilution. Too much secondary antibody can result in nonspecific bands. Incubate the blot at room temperature for 1–2 h with gentle agitation. Table 1 shows multiple options for secondary detection reagents and methods. More details are available in the Immunoblot Data Analysis section below.
Slot/Dot Blot
The procedure is similar to the procedure for 1D immunoblotting, but differs because protein samples are not separated electrophoretically but are spotted directly onto the membrane either manually or by use of a blotting unit (dot or slot blot format).
procedure using manual spotting
Handle manual spotting as follows. Place a dry filter paper on a stack of dry paper towels. Place filter paper that is pre-wet with transfer buffer on top of the dry filter paper. Place a pre-wet membrane on top of the pre-wet filter paper. Samples are spotted onto the pre-wet membrane and are allowed to absorb into the membrane. After the sample is absorbed, place the membrane on a clean, dry filter paper to dry.
procedure using a vacuum-blotting unit
Analysts typically use a vacuum-blotting unit as follows. Prepare a membrane, and place it in the blotting unit according to the manufacturer's instructions. Apply vacuum to the blotting unit to remove excess buffer. To improve solubility, dissolve the sample in a buffer, and if it is not clear, remove precipitates by centrifugation. If the sample is too viscous to pipet, then dilute it further with buffer. With the vacuum off, carefully pipet samples into the wells, and apply vacuum to the blotting unit. After all the samples have filtered through the membrane, turn off the vacuum, add buffer to each well to wash down the sides, and apply vacuum again. Remove the membrane, and proceed with immunoblotting.
2D Immunoblotting
sample preparation
The compounds used to solubilize proteins must not increase the ionic strength of the solution. For example, a common sample solubilization solution is the following: 8 M urea, 50 mM dithiothreitol (DTT) or 2 mM tributyl phosphine (TBP), 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 0.2% carrier ampholytes, and 0.0002% bromophenol blue. The addition of carrier ampholytes enhances the solubility of proteins as they approach their isoelectric points. The use of ampholytes produces an approximately uniform conductivity across the pH gradient without affecting its shape, meaning that the concentration of carrier ampholytes should be optimized.
charge separation
Several vendors produce and sell immobilized pH gradient (IPG) strips, or they can be made in-house according to  1054
1054 . The choice of IPG strips depends on the pI of the proteins of interest. The size of the IPG should match the size of the second-dimension gel. The amount of protein in each sample should be determined, and the amounts loaded on the IPG strips should be in the range of 10–300 µg, depending on the size of the IPG. The sample and standard should be loaded according to the manufacturer's instructions or according to
. The choice of IPG strips depends on the pI of the proteins of interest. The size of the IPG should match the size of the second-dimension gel. The amount of protein in each sample should be determined, and the amounts loaded on the IPG strips should be in the range of 10–300 µg, depending on the size of the IPG. The sample and standard should be loaded according to the manufacturer's instructions or according to  1054
1054 . Analysts then proceed with the isoelectric focusing by applying the electrical parameters described in
. Analysts then proceed with the isoelectric focusing by applying the electrical parameters described in  1054
1054 or by the manufacturer.
or by the manufacturer.
molecular weight separation
After charge separation, analysts must equilibrate the strip in SDS-containing buffer before separation in the second dimension to determine molecular weight (as described previously in the 1D Immunoblotting section). Analysts should position the strip directly on top of the gel, then secure the strip by overlaying it with 0.5%–1.0% agarose prepared in SDS–PAGE running buffer. To track the ion front in the second dimension, analysts can add bromophenol blue to the agarose.
IMMUNOBLOT DATA ANALYSIS
The presence or absence of bands usually is determined by comparison to a control (highly characterized antigens known or qualified to give a precise or expected response) of a type that is similar to the antigen being processed. Although analysts usually perform a qualitative comparison, bands or dots can be quantitated using a detection system (e.g., after incubating in a solution containing 4-CN peroxidase substrate; also see Table 1), and are compared to the control bands run in parallel (e.g., in the same gel).
Detection Options
enhanced chemiluminescence
Enhanced chemiluminescence is a popular method for detection in immunoblot analysis because it is highly sensitive (detection to pg or lower levels), and can be used to quantitate the relative concentration of the protein of interest. The method depends on incubation of the blot with a substrate that luminesces when exposed to the reporter on the secondary antibody. The light is detected using either photographic film or a charge-coupled device (CCD) camera. The image then is analyzed by densitometry to evaluate the relative amount of protein staining in terms of optical density. By using an appropriate set of molecular weight standards as markers, analysts can estimate molecular weight.
fluorescence detection
Direct fluorescence can be used to detect proteins on blots. Direct fluorescence is simple, rapid, sensitive, and has a greater linear range than enhanced chemiluminescent detection. The advantage of direct fluorescence is the ability to detect many different fluorescent signals. This analysis avoids the need to reprobe the blot. Compared to enhanced chemiluminescence, fluorescence methods are easier to visualize and quantitate on CCD or laser-scanning imaging systems. Some data-acquisition systems permit extending the time of data acquisition to optimize signal-to-noise levels. Fluorescence-labeled blots that can be re-examined are useful for this purpose.
Enhanced chemifluorescence (ECF) is another common fluorescence method. ECF uses secondary antibodies conjugated to either HRP or AP. The enzyme-conjugated antibodies react with specific substrates that produce fluorescence after enzymatic cleavage. Analysts visualize the resulting signals using UV epi-illumination and capture digital images. An ECF signal has a greater linear range than traditional enhanced chemiluminescence. For example, direct fluorescence has a limit of detection in the pg range, and also has about 2 logs of linear dynamic range.
Quantum dots also are an alternative to detect proteins in immunoblot analysis. Quantum dots are a type of probe that can be conjugated to antibodies simultaneously or sequentially to detect multiply labeled antigens, without the need for blot stripping. Similarly, near-infrared (NIR) fluorophore–linked antibody is a method for antibody detection whereby light produced from the excitation of a fluorescent dye is measured in a static state. Light measured in a static state allows more precise and accurate detection than light measured in a dynamic state (e.g., chemiluminescence).
radioactive detection
Proteins also can be detected by labeling an antigen with a radioactive isotope (e.g., iodine). On the one hand, this method has the advantage that the radioactivity in a band is easy to quantitate by means of time exposure to film and densitometry, or by directly excising the band from the membrane and counting using a scintillation counter. On the other hand, radioactivity also introduces the disadvantage of safety because analysts must manage radioactive material, and analytical laboratories must have a program to control and monitor waste management and individual exposure.
Immunoblot Quantitation
nonelectrophoretic quantitation
The quantitation of a specific protein is achieved when the blot procedure is properly optimized and generates a linear response range over a particular time frame. Immunoblot quantitation includes several elements: adequate antigen and antibody concentrations and purity, antibody specificity, blocking conditions, sufficient washes, and the duration and intensity of the signals. Once the exposures are captured on a film or electronically under optimized conditions, analysts use densitometric methods to quantitate results by comparing a specific protein on the blot and on the standard. Analysts can correct results for background by including a negative control.
The intensity of the bands depends on the amount of protein. Different commercial software packages are available for image analysis of bands on a film. Alternatively, digital imaging systems containing CCD cameras usually include software designed to perform data analysis.
electrophoretic quantitation
Proteins of various molecular weights are identified by the extrapolation of plots of relative mobilities of prestained proteins of known molecular weight and can be compared to the positive control. Positive controls are trended to determine the limit range of the densitometry results compared to the nominal concentration results. Independent of the detection method, the following criteria must be met for a valid Western blot result.
- Ensure adequate development by minimizing membrane overexposure and visualizing staining controls.
- The prestained molecular weight markers must be visible and must cover the anticipated range.
- The band(s) should have the appropriate location and intensity for the standard, the control, and the protein of interest.
- There should be no blot or staining artifacts that obscure the visualization and interpretation of bands.
METHOD VALIDATION
As outlined by ICH Guideline Q2(R1) Validation of Analytical Procedures: Text and Methodology (effective November 2005) and USP general chapter Validation of Compendial Procedures  1225
1225 , a qualitative assay such as the slot/dot blot requires validation of specificity. Specificity is the ability to detect the analyte in the presence of other components. For validation, it should be shown that the particular steps of the slot/dot blot method can detect the antigen when present and do not report false positive results when the antigen is absent. In addition, demonstration of the specificity of the antigen-specific antibodies is part of the specificity evaluation.
, a qualitative assay such as the slot/dot blot requires validation of specificity. Specificity is the ability to detect the analyte in the presence of other components. For validation, it should be shown that the particular steps of the slot/dot blot method can detect the antigen when present and do not report false positive results when the antigen is absent. In addition, demonstration of the specificity of the antigen-specific antibodies is part of the specificity evaluation.
USP general chapter  1225
1225 provides guidelines for the validation of analytical procedures, and analysts should consider this resource when they validate immunoblot methods. All methods require a demonstration of the specificity of the antibody to the antigen and the lack of recognition of other proteins and reagents in the matrix. Identity tests require only specificity. Limit tests require specificity and LOD. A sensitivity control incorporated into each test can show that the LOQ is met on each determination to account for potential changes in the sensitivity of the method. A quantitative test requires all ICH validation parameters, including robustness testing.
provides guidelines for the validation of analytical procedures, and analysts should consider this resource when they validate immunoblot methods. All methods require a demonstration of the specificity of the antibody to the antigen and the lack of recognition of other proteins and reagents in the matrix. Identity tests require only specificity. Limit tests require specificity and LOD. A sensitivity control incorporated into each test can show that the LOQ is met on each determination to account for potential changes in the sensitivity of the method. A quantitative test requires all ICH validation parameters, including robustness testing.
Demonstration of electrophoretic immunoblot specificity should include the following: stained gels to show protein separation, stained blots to show adequate protein transfer to the membrane, blots with control samples to show the specificity of the conjugate to the primary antibody, and blots that show the binding of the antibody to the appropriate antigen. Method validation also can identify the need for control membranes for each assay, as well as protein sensitivity controls as measures of system suitability.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Maura C Kibbey, Ph.D.
Senior Scientific Liaison, Biologics & Biotechnology (301) 230-6309 |
(GCBA2010) General Chapters - Biological Analysis |
USP38–NF33 Page 1135
Pharmacopeial Forum: Volume No. 38(4)