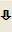- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Gentamicin Sulphate |
 |
(Ph Eur monograph 0331)
1405-41-0
Aminoglycoside antibacterial.
Gentamicin and Hydrocortisone Acetate Ear Drops
Ph Eur
Mixture of the sulphates of antimicrobial substances produced by Micromonospora purpurea, the main components being gentamicins C1, C1a, C2, C2a and C2b.
Minimum 590 IU/mg (anhydrous substance).
White or almost white, hygroscopic powder.
Freely soluble in water, practically insoluble in alcohol.
First identification C, D.
Second identification A, B, D.
A. Dissolve about 10 mg in 1 ml of water R and add 5 ml of a 400 g/l solution of sulphuric acid R. Heat on a water-bath for 100 min, cool and dilute to 25 ml with water R. Examined between 240 nm and 330 nm (2.2.25), the solution shows no absorption maximum.
B. Thin-layer chromatography (2.2.27).
Test solution Dissolve 25 mg of the substance to be examined in water R and dilute to 5 ml with the same solvent.
Reference solution Dissolve the contents of a vial of gentamicin sulphate CRS in water R and dilute to 5 ml with the same solvent.
Plate TLC silica gel plate R.
Mobile phase The lower layer of a mixture of equal volumes of concentrated ammonia R, methanol R and methylene chloride R.
Application 10 µl.
Development Over 2/3 of the plate.
Drying In air.
Detection Spray with ninhydrin solution R1 and heat at 110 °C for 5 min.
Results The 3 principal spots in the chromatogram obtained with the test solution are similar in position, colour and size to the 3 principal spots in the chromatogram obtained with the reference solution.
C. Examine the chromatograms obtained in the test for composition.
Results The chromatogram obtained with the test solution shows 5 principal peaks having the same retention times as the 5 principal peaks in the chromatogram obtained with reference solution (a).
D. It gives reaction (a) of sulphates (2.3.1).
Dissolve 0.8 g in carbon dioxide-free water R and dilute to 20 ml with the same solvent.
Solution S is clear (2.2.1) and not more intensely coloured than intensity 6 of the range of reference solutions of the most appropriate colour (2.2.2, Method II).
3.5 to 5.5 for solution S.
+ 107 to + 121 (anhydrous substance).
Dissolve 2.5 g in water R and dilute to 25.0 ml with the same solvent.
Liquid chromatography (2.2.29): use the normalisation procedure taking into account only the peaks due to gentamicins C1, C1a, C2, C2a and C2b; use the chromatogram supplied with gentamicin sulphate CRS to identify the corresponding peaks.
Test solution Dissolve 50 mg of the substance to be examined in the mobile phase and dilute to 100.0 ml with the mobile phase.
Reference solution (a) Dissolve the content of a vial of gentamicin sulphate CRS in the mobile phase and dilute with the mobile phase to obtain a solution containing 0.5 mg/ml.
Reference solution (b) Dilute 5.0 ml of reference solution (a) to 100.0 ml with the mobile phase.
- — size: l = 0.25 m, Ø = 4.6 mm,
- — stationary phase: styrene-divinylbenzene copolymer R (8 µm) with a pore size of 100 nm,
- — temperature: 55 °C.
Mobile phase A mixture prepared with carbon dioxide-free water R containing 60 g/l of anhydrous sodium sulphate R, 1.75 g/l of sodium octanesulphonate R, 8 ml/l of tetrahydrofuran R, 50 ml/l of 0.2 M potassium dihydrogen phosphate R previously adjusted to pH 3.0 with dilute phosphoric acid R and degassed.
Flow rate 1.0 ml/min.
Post-column solution A carbonate-free sodium hydroxide solution R diluted 1 to 25, previously degassed, which is added pulse-less to the column effluent using a 375 µl polymeric mixing coil.
Flow rate 0.3 ml/min.
Detection Pulsed amperometric detector or equivalent with a gold indicator electrode, a silver-silver chloride reference electrode, and a stainless steel auxiliary electrode which is the cell body, held at respectively + 0.05 V detection, + 0.75 V oxidation and - 0.15 V reduction potentials, with pulse durations according to the instrument used.
Injection 20 µl.
Run time 1.2 times the retention time of gentamicin C1.
System suitability Reference solution (a):
- — peak-to-valley ratio: minimum 2.0 where Hp = height above the baseline of the peak due to gentamicin C2a, and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to gentamicin C2.
- — gentamicin C1 : 20.0 per cent to 40.0 per cent,
- — gentamicin C1a : 10.0 per cent to 30.0 per cent,
- — sum of gentamicins C2, C2a, and C2b: 40.0 per cent to 60.0 per cent,
- — disregard limit: the area of the peak due to gentamicin C1a in the chromatogram obtained with reference solution (b).
Liquid chromatography (2.2.29) as described in the test for composition.
- — any impurity: maximum 3.0 per cent,
- — total: maximum 10.0 per cent.
Maximum 1.0 per cent.
32.0 per cent to 35.0 per cent (anhydrous substance).
Dissolve 0.250 g in 100 ml of distilled water R and adjust the solution to pH 11 using concentrated ammonia R. Add 10.0 ml of 0.1 M barium chloride and about 0.5 mg of phthalein purple R. Titrate with 0.1 M sodium edetate, adding 50 ml of alcohol R when the colour of the solution begins to change and continue the titration until the violet-blue colour disappears.
1 ml of 0.1 M barium chloride is equivalent to 9.606 mg of SO4.
Maximum 15.0 per cent, determined on 0.300 g.
Maximum 1.0 per cent, determined on 0.50 g.
Less than 0.71 IU/mg, if intended for use in the manufacture of parenteral dosage forms without a further appropriate procedure for the removal of bacterial endotoxins.
Carry out the microbiological assay of antibiotics (2.7.2).
In an airtight container. If the substance is sterile, store in a sterile, airtight, tamper-proof container.
Specified impurities A, B, C.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) : D, E.
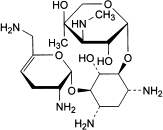
A. 2-deoxy-4-O-[3-deoxy-4-C-methyl-3-(methylamino)-β-l-arabinopyranosyl]-6-O-(2,6-diamino-2,3,4,6-tetradeoxy-α-d-glycero-hex-4-enopyranosyl)-l-streptamine (sisomicin),
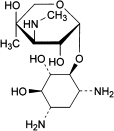
B. 2-deoxy-4-O-[3-deoxy-4-C-methyl-3-(methylamino)-β-l-arabinopyranosyl]-l-streptamine (garamine),
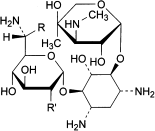
C. R = CH3, R′ = OH: 4-O-(6-amino-6,7-dideoxy-d-glycero-α-d-gluco-heptopyranosyl)-2-deoxy-6-O-[3-deoxy-4-C-methyl-3-(methylamino)-β-l-arabinopyranosyl]-d-streptamine (gentamicin B 1),
D. R = H, R′ = NH2: 2-deoxy-4-O-[3-deoxy-4-C-methyl-3-(methylamino)-β-l-arabinopyranosyl]-6-O-(2,6-diamino-2,6-dideoxy-α-d-gluco-hexopyranosyl)-l-streptamine,
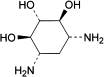
E. 2-deoxystreptamine.
Ph Eur